Airway and Anesthesia Devices Pipeline Report including Stages of Development, Segments, Region and Countries, Regulatory Path and Key Companies, 2022 Update
Powered by ![]()
All the vital news, analysis, and commentary curated by our industry experts.
Airway and Anesthesia Devices Pipeline Product Market Report Overview
Airway and Anesthesia Devices are single-use devices used in conjunction with anesthesia equipment to administer anesthesia. The Airway and Anesthesia Devices pipeline market research report provides comprehensive information about the Airway and Anesthesia Devices pipeline products with a comparative analysis of the products at various stages of development and information about the clinical trials which are in progress.
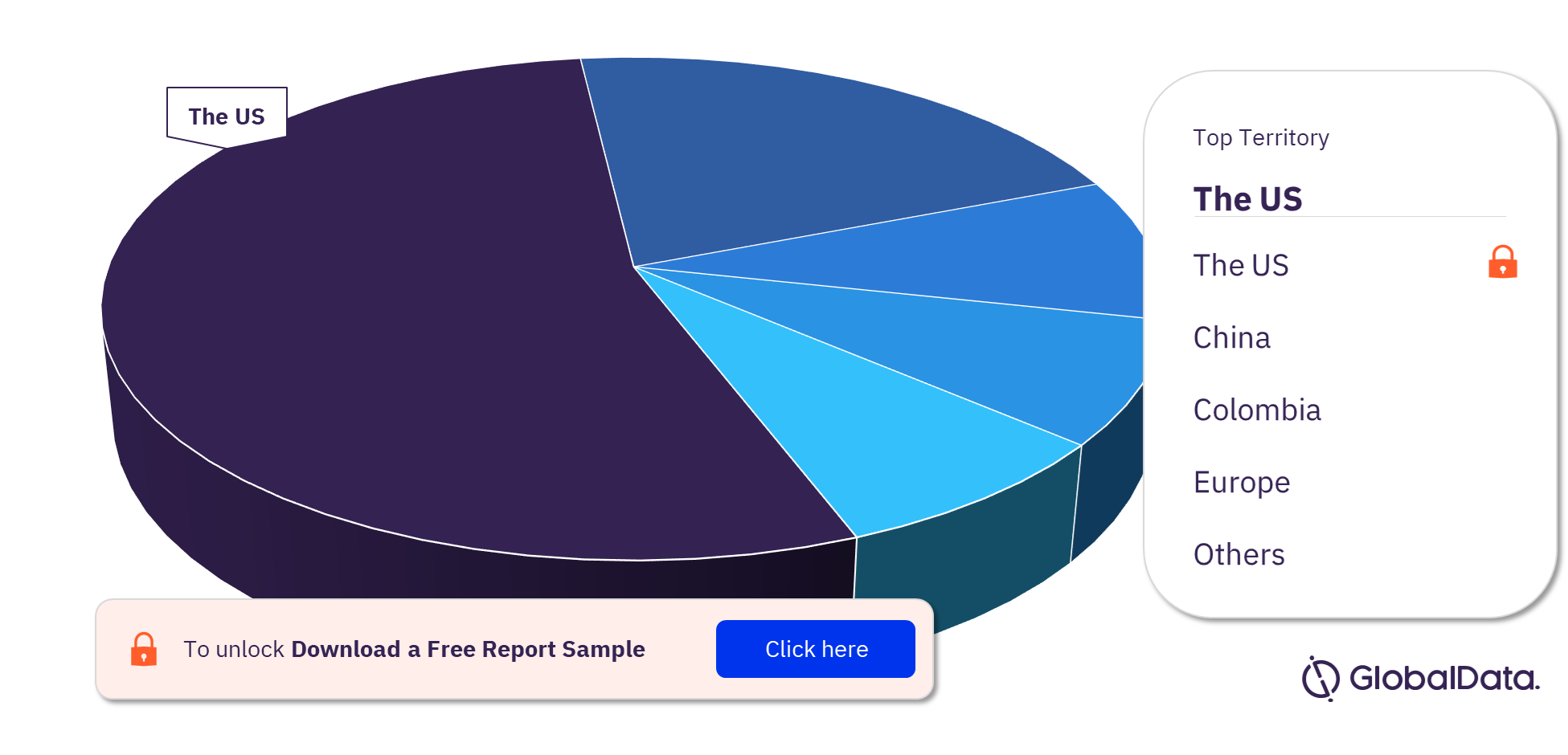
Airway and Anesthesia Devices Pipeline Products Market Segmentation by Territories
Some of the key territories with products in the pipeline are the US, China, Colombia, Europe, Canada, Italy, Oman, Poland, Switzerland, the UAE, and the UK. As of December 2022, the US has the highest number of products in the pipeline out of them all.
Airway and Anesthesia Devices Pipeline Products Market Analysis, by Territories, 2022 (%)
For more territory insights into the Airway and Anesthesia Devices pipeline products market, download a free report sample
Airway and Anesthesia Devices Pipeline Products Market Segmentation by Key Regulatory Paths
The key regulatory paths followed by the Airway and Anesthesia Devices pipeline products market are 510(k), CE, NMPA, UKCA, EUA, INVIMA, and MDL. Most of the products follow the 510(k) pathway to enter the market.
Airway and Anesthesia Devices Pipeline Products Market Analysis, by Regulatory Paths, 2022 (%)
For more Airway and Anesthesia Devices pipeline products regulatory path insights, download a free report sample
Airway and Anesthesia Devices Pipeline Products Market - Competitive Landscape
Some of the leading companies in the Airway and Anesthesia Devices pipeline products market are Adroit USA, Inc, Airway Innovations LLC, Cryofocus Medtech Shanghai Co Ltd, DMF Medical Inc, Drexel University, H. Lee Moffitt Cancer Center & Research Institute Inc, Johns Hopkins University, Medical Device Investments, Inc., Rutgers the State University of New Jersey, and Sedana Medical AB.
Cryofocus Medtech Shanghai Co Ltd: It is a medical device company that engages in the research, development, and manufacturing of cryoablation systems for cardiovascular interventional therapy. Cryofocus Medtech is headquartered in Shanghai, China.
Drexel University: It is a research university which provides educational degrees and research programs. The university offers courses for undergraduate courses, graduate and professional courses, adult education programs, colleges and schools, libraries, and online training services. Drexel is headquartered in Philadelphia, Pennsylvania, the US.
H. Lee Moffitt Cancer Center & Research Institute Inc: It is a healthcare service provider that offers medical services such as surgical care, fertility preservation, radiation therapy, interventional radiology, rehabilitation services, chemotherapy, cardio-oncology, blood and bone marrow transplant, cancer survivorship clinic, tumor board, interventional pain management, supportive care medicine, survivorship program, clinical trials, and others. Moffitt is headquartered in Tampa, Florida, the US.
Airway and Anesthesia Devices Pipeline Products Market Report Overview
| Key Territories | The US, China, Colombia, Europe, Canada, Italy, Oman, Poland, Switzerland, the UAE, and the UK |
| Key Regulatory Paths | 510(k), CE, NMPA, UKCA, EUA, INVIMA, and MDL |
| Leading Companies | Adroit USA, Inc, Airway Innovations LLC, Cryofocus Medtech Shanghai Co Ltd, DMF Medical Inc, Drexel University, H. Lee Moffitt Cancer Center & Research Institute Inc, Johns Hopkins University, Medical Device Investments, Inc., Rutgers the State University of New Jersey, and Sedana Medical AB |
Segments Covered in the Report
Airway and Anesthesia Devices Pipeline Products Market Territories Outlook
- The US
- China
- Colombia
- Europe
- Canada
- Italy
- Oman
- Poland
- Switzerland
- The UAE
- The UK
Airway and Anesthesia Devices Pipeline Products Market Regulatory Paths Outlook
- 510(k)
- CE
- NMPA
- UKCA
- EUA
- INVIMA
- MDL
Scope
This report provides:
- Extensive coverage of the Airway and Anesthesia Devices under development.
- Details of major pipeline products which include product description, licensing, collaboration details, and other developmental activities.
- Reviews of the major players involved in the development of Airway and Anesthesia Devices and lists all their pipeline projects.
- The coverage of pipeline products based on various stages of development ranging from early development to the approved/issued stage.
- Key clinical trial data of ongoing trials specific to pipeline products.
- Recent developments in the segment/industry.
Reasons to Buy
The report enables you to:
- Formulate significant competitor information, analysis, and insights to improve R&D strategies
- Identify emerging players with potentially strong product portfolios and create effective counter strategies to gain a competitive advantage
- Identify and understand important and diverse types of Airway and Anesthesia Devices under development
- Develop market-entry and market-expansion strategies
- Plan mergers and acquisitions effectively by identifying major players with the most promising pipeline
- In-depth analysis of the product’s current stage of development, territory, and estimated launch date
Airway Innovations LLC
Cryofocus Medtech Shanghai Co Ltd
DMF Medical Inc
Drexel University
H. Lee Moffitt Cancer Center & Research Institute Inc
Johns Hopkins University
Medical Device Investments, Inc.
Rutgers The State University of New Jersey
Sedana Medical AB
Teleflex Inc
University of South Florida
Vida Medical Devices Inc
Vincent Medical Holdings Ltd
Wake Forest Baptist Medical Center
Xhale Inc
Table of Contents
Table
Figures
Frequently asked questions
-
What are the key territories in the Airway and Anesthesia Devices pipeline products market?
The US, China, Colombia, Europe, Canada, Italy, Oman, Poland, Switzerland, the UAE, and the UK are some of the key territories with products in the pipeline.
-
What are the key regulatory paths of the Airway and Anesthesia Devices pipeline products market?
Some of the key regulatory paths followed by the Airway and Anesthesia Devices pipeline products market are 510(k), CE, NMPA, UKCA, EUA, INVIMA, and MDL.
-
Which regulatory path is the most followed to enter the Airway and Anesthesia Devices pipeline products market?
Most of the pipeline products follow the 510(k) pathway to enter the market.
-
What are the leading companies in the Airway and Anesthesia Devices pipeline products market?
Some of the leading companies in the Airway and Anesthesia Devices pipeline products market are Adroit USA, Inc, Airway Innovations LLC, Cryofocus Medtech Shanghai Co Ltd, DMF Medical Inc, Drexel University, H. Lee Moffitt Cancer Center & Research Institute Inc, Johns Hopkins University, Medical Device Investments, Inc., Rutgers the State University of New Jersey, and Sedana Medical AB.
Get in touch to find out about multi-purchase discounts
reportstore@globaldata.com
Tel +44 20 7947 2745
Every customer’s requirement is unique. With over 220,000 construction projects tracked, we can create a tailored dataset for you based on the types of projects you are looking for. Please get in touch with your specific requirements and we can send you a quote.
Related reports
View more Anesthesia and Respiratory Devices reports